
Radioactive isotopes that decay by pure electron capture can be inhibited from radioactive decay if they are fully ionized ("stripped" is sometimes used to describe such ions). The electron that is captured is one of the atom's own electrons, and not a new, incoming electron, as might be suggested by the way the above reactions are written. Two diagrams comprise the leading (second) order, though as a virtual particle, the type (and charge) of the W-boson is indistinguishable. An electron interacts with an up quark in the nucleus via a W boson to create a down quark and electron neutrino. The leading-order Feynman diagrams for electron capture decay. Alvarez went on to study electron capture in gallium ( 67 K-electron capture was first observed by Luis Alvarez, in vanadium, 48 The theory of electron capture was first discussed by Gian-Carlo Wick in a 1934 paper, and then developed by Hideki Yukawa and others. For example, rubidium-83 (37 protons, 46 neutrons) will decay to krypton-83 (36 protons, 47 neutrons) solely by electron capture (the energy difference, or decay energy, is about 0.9 MeV). If the energy difference between the parent atom and the daughter atom is less than 1.022 MeV, positron emission is forbidden as not enough decay energy is available to allow it, and thus electron capture is the sole decay mode. Electron capture is sometimes called inverse beta decay, though this term usually refers to the interaction of an electron antineutrino with a proton. In nuclear physics, beta decay is a type of radioactive decay in which a beta ray (fast energetic electron or positron) and a neutrino are emitted from an atomic nucleus. Electron capture is sometimes included as a type of beta decay, because the basic nuclear process, mediated by the weak force, is the same.
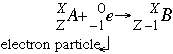
Electron capture is always an alternative decay mode for radioactive isotopes that do have sufficient energy to decay by positron emission. However, a positive atomic ion may result from further Auger electron emission.Įlectron capture is an example of weak interaction, one of the four fundamental forces.Įlectron capture is the primary decay mode for isotopes with a relative superabundance of protons in the nucleus, but with insufficient energy difference between the isotope and its prospective daughter (the isobar with one less positive charge) for the nuclide to decay by emitting a positron. Simple electron capture by itself results in a neutral atom, since the loss of the electron in the electron shell is balanced by a loss of positive nuclear charge. Electron capture sometimes also results in the Auger effect, where an electron is ejected from the atom's electron shell due to interactions between the atom's electrons in the process of seeking a lower energy electron state.įollowing electron capture, the atomic number is reduced by one, the neutron number is increased by one, and there is no change in mass number. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion.įollowing capture of an inner electron from the atom, an outer electron replaces the electron that was captured and one or more characteristic X-ray photons is emitted in this process.

The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy.

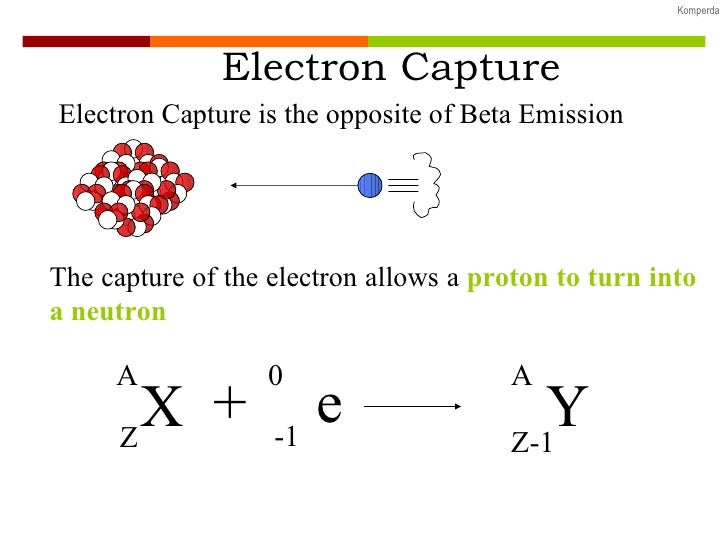
This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.Į or when written as a nuclear reaction equation, e − 1 0 + p 1 1 ⟶ n 0 1 + 0 0 The outer electron is ejected from the atom, leaving a positive ion.Įlectron capture ( K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Lower right: In the Auger effect, the energy absorbed when the outer electron replaces the inner electron is transferred to an outer electron. An x-ray, equal in energy to the difference between the two electron shells, is emitted. Lower left: An outer electron replaces the "missing" electron.


 0 kommentar(er)
0 kommentar(er)
